日前,公司岩石矿物材料研究团队与中国地质大学(北京)等单位合作在环境领域国际期刊Journal of Cleaner Production(2021年中科院分区:环境科学与生态学大类一区TOP)在线发表了题为“Activate nutrients access to plants as multiphase slow-release fertilizers by sintering reaction in K2O–MgO–Al2O3–SiO2 system using K-feldspar as a major source”(https://doi.org/10.1016/j.jclepro.2022.133511)的研究论文,第一作者为陶隆凤老师,通讯作者为刘昶江老师,研究生郭玲参与相关研究工作,第一单位为河北省岩石矿物材料绿色开发重点实验室。
该论文报道了以钾长石、菱镁矿等天然矿物为原料直接制备矿物型复合缓释肥料的系统研究,并制备了不同系列钾-铝-硅和钾-镁-硅矿物型复合缓释肥料、表征了其养分释放能力,其创新点在于将天然矿物中的营养元素直接转变为可供植物吸收的状态,并以特定的“缓释肥料”形式存在与矿物骨架中,以进一步提升养分利用效率。
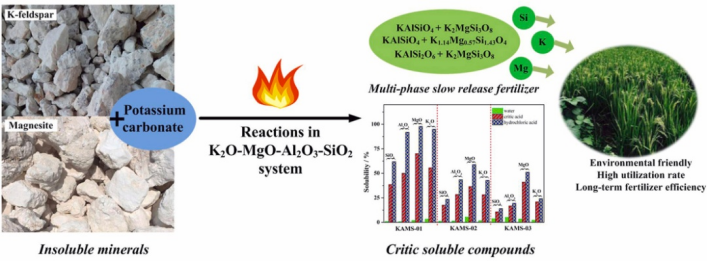
图1论文图示摘要
论文摘要:The sintering reactions inK2O-MgO-Al2O3-SiO2 system using K-feldspar,magnesite, andpotassium carbonate as the starting materials were studied to prepare potassium magnesium silicates and potassium aluminosilicates asmultiphase slow-release fertilizers (SRFs). Effects of sintering temperature (850-1250 °C), time (1-5 h), andaddition of K2CO3(100%-130%) on the reaction extents as well as the types of final products wereinvestigated. Results showed that the multi-phases ofK1.14Mg0.57Si1.43O4 andKAlSiO4wereprepared asstable products underthe concerned conditions of1050 °C for 2h, andK2MgSiO4 was obtained instead ofK1.14Mg0.57Si1.43O4 under thesituation of adding excessive K2CO3.Sintering experiments of fiveraw mixtures were designed to discuss reaction mechanism by comparing the Al-phases infinalproducts.The results indicated that Al2O3 componentfavored to persist aspotassium aluminosilicates (KAlSiO4or KAlSi2O6),andthree multiphase compounds,KAlSiO4 +K1.14Mg0.57Si1.43O4,KAlSiO4 +K2MgSi3O8, andKAlSi2O6 + K2MgSi3O8 were successfully prepared at optimal conditionsandsubsequently characterized.Solubility tests of sintered samples in water,20 g/L critic acid solution, and0.5 mol/L hydrochloric acid solution showed thatthe three samplesexhibited increasing solubility orderly with consistent component soluble sequence of MgO > K2O≥Al2O3> SiO2 in differentsolutions.K1.14Mg0.57Si1.43O4 is found to perform the best solublebehavior, whileKAlSi2O6 shows aless solublebehavior. Present work proposed considerable approaches to make theinert elements in minerals (K-feldspar and magnesite)and the active element (K) in potassium carbonateaccessible for plants in a slow-releaseroute.
